Atoms To Mass In Grams Converter To Cups
Write on a piece of paper the name of an element that interests you and the number of atoms of that element you wish to convert to grams. For example, you write 'Seven atoms of lithium.'
Convert Atoms To Mass In Grams
Find the element that interests you in an online periodic table (see Resources) or any chemistry textbook. For example, you find the element lithium (Li) in the first column of the periodic table, second from the top. Read the number under the symbol for lithium.


For example, you read 6.941. Divide the number under the symbol of the element by 6.022 x 10^23 using a scientific calculator. For example, 6.941/(6.022 x 10^23) = 1.152 x 10^-23. Multiply your answer times the number of atoms you wrote on the piece of paper. For example, (1.152 x 10^-23) x 7 = 8.068 x 10^-23.
What is the equation to convert grams into milliliters? Grams is a unit of mass. Is a unit of volume, which can be roughly converted if talking about.
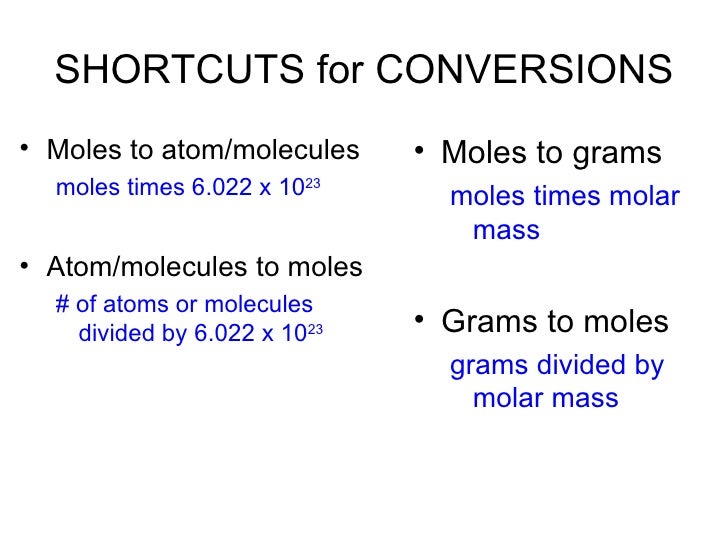
Seven atoms of lithium weigh approximately 8.07 x 10^-23 grams.